usp class vi compliant
Many plastics manufacturers find it advantageous to have their materials classified especially if their plastic resins are a likely candidate to be used in medical devices. Compounds made without animal-derived ingredients BSETSE concerns.
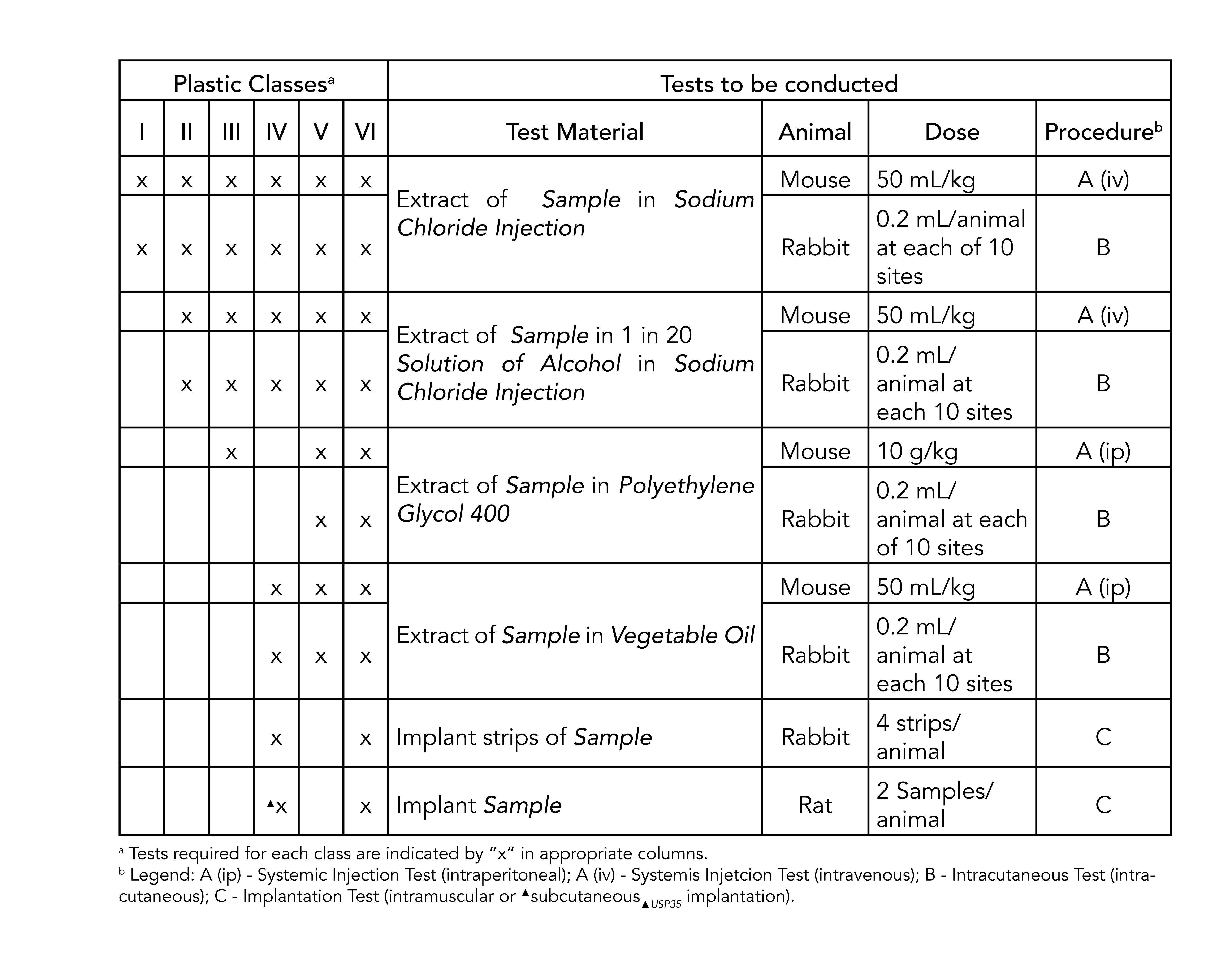
Class VI Test USP Project Number.

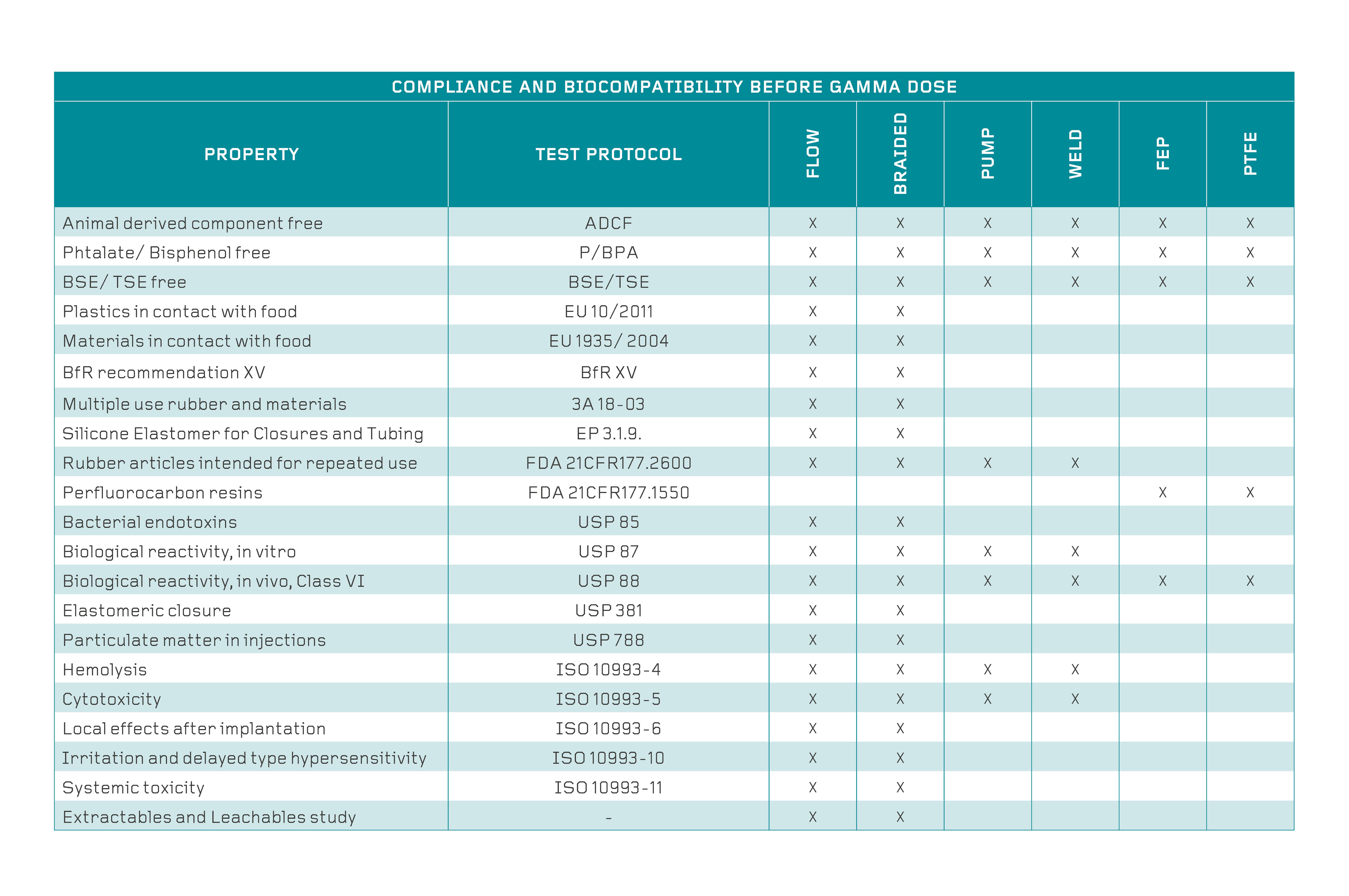
. USP Class VI and FDA White List Silicone and Organic Elastomer Compounds for. USP Class VI compliant compounds are formulated with ingredients providing certifiable biocompatibility and low extractables for system toxicity and purity. These CPC quick-disconnect fittings feature an automatic shutoff valve which closes off the flow path at disconnection protecting valuable media while eliminating the need.
That being said if you cant get an ISO 10993 compliant material often because the material simply hasnt been tested using a USP Class VI material is a less risky option. USP Class VI Certificate of Compliance. Table 1 shows our standard programme FDA compliant com-pounds which can be produced in a few days.
RoHS a European Union Directive restricts the use of certain substances but manufacturers also need to know whether all the ingredients in a medical silicone are made of compliant materials. Sil 714001 USP class VI Silicone 1 70 Yes transl. Sheet material is available in various thick-nesses and with a standard of 36 width.
What is ADI-Free BSE-Free TSE-Free. 711 Systemic and Intracutaneous Testing Preparation. Overview of USP Class VI Approved Plastic Materials.
The test article was. When production of the elastomer contain no ADI with respect to source manufacture and treatment they cannot. ADI-free certifies that the raw materials used in production of the elastomer contain no Animal Derived Ingredients ADI.
Material is ordered by the linear inch and is most suited for die cutting parts. Since lives are routinely on the line manufacturers of medical devices have to plan for every possible use case scenario and create products that address those situations. Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials.
High quality USP Class VI compliant sheet material made from our own specially formulated compounds. 157 Charles Colman Boulevard Pawling NY 12564. Specially formulated for long term sealing.
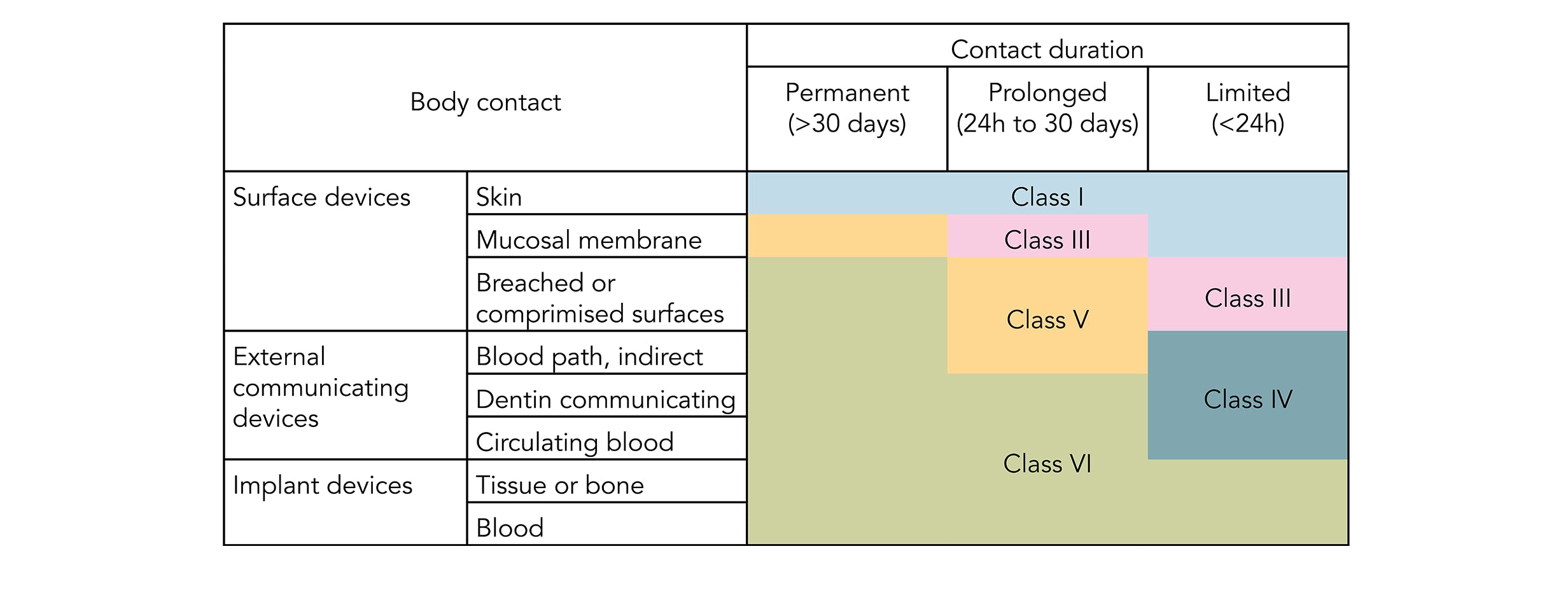
USP Class VI Medical Packaging How to Ensure Compliance when Heat Sealing When it comes to regulatory compliance medical devices are in a league of their own. So here is a new one - a customer has requested us to conduct testing compliant to USP Class VI and ISO10993-1 compliant. The USP defines six plastics classes from class I to class VI with class VI being the most rigorous and most frequently requested certification.
Master Bond systems are very versatile and can be used for both disposable and reusable medical devices. EPDM red Silicone and VitonTM. All these special grade products have passed this rigorous test.
USP Class VI materials EPDM Silicone Fluorocarbon and Perfluoroelastomer 24 materials which are compliant to FDA 21 CF R1772600. This is the second material in our SSP2390 family. United States Pharmacopeia USP 26 NF21 2003 Class VI.
Specifically USP publishes test instructions for the plastics polymers and elastomers that are used in medical devices and surgical equipment. Specially formulated for long term sealing. Some medical silicones must meet USP Class VI FDA CFR 21 1772600 and RoHS requirements.
IEGeek - 2006. Pharmacopeia USP a non-profit organization whose standards inform decision-making at the US. Pharmacopoeia Class VI judges the suitability of plastic material intended for use as containers or accessories for parenteral preparations.
Suitability under USP Class VI is typically a. Pharmacopoeia USP Class VI requirements. USP Class VI materials are available in 70 durometer EPDM Silicone and Viton.
Body and seal materials are made of USP Class VI-compliant and BSETSE-free materials. Has a full range of specialty adhesives epoxies primers for polyolefins UV curables and silicones that have been fully tested to meet USP Class VI requirements. There are six classes VI being the most rigorous.
I know that performing a USP Class VI test even for a 30 day period will still not perform to ISO10993-1 per General ProgramBluebook Memo G95-1 We are. EPFEPerfluoroelastomer Seals Special RubberFabrications. Phone 845 855-1000 800 431-0101 Fax 845 855-1139.
Compliance to USP Class VI is often requested by end users. Fitting body constructed of durable polysulfone. Testing for compliance involves an assessment of the effects of the material and extractables on tissue.
USP Class Testing standards are determined by the United States. USP Class VI compliant Extrusions Cord. Sil 714002 USP class VI Silicone 1 70 Yes transl.
Food and Drug Administration FDA. Pawling Engineered Products Inc. Specialty Silicone Products SSP recently received a certificate of compliance COC from NAMSA the worlds leading MedTech Contract Research Organization for a 10-durometer silicone test article that passed USP Biological Reactivity Tests In Vivo for USP Plastics Class VI.
USP Class VI refers to a set of biocompatibility testing requirements from the US. Watershed 11122XC 70 EXPERIMENTAL DESIGN AND DOSAGE 71 Preparation of Test and Control Articles. Manufactures standard and custom size o-rings compliant to the US.
An article of commerce that is recognized in the USPNF complies with USPNF standards when it meets all of the requirements stated in the articles monograph applicable General Chapters and the General Notices with monograph requirements superseding those of the General Chapters and General Notices in any cases where requirements differ. While it is possible a USP Class VI material could also be ISO 10993 compliant its not a given and USP Class VI alone is not sufficient for adherence to ISO 10993. 7111 The test article 60 cm2 was combined with 10 mL of vehicle at a ratio of 120 cm2 per 20 mL per USP guidelines.
Compliance to USP Class VI is often requested by users in the biopharmaceutical and medical industries. 7 USP Class VI materials EPDM silicone fluorocarbon and perfluoroelastomer 24 materials which are compliant to FDA 21 CFR1772600. Valves restrict flow when fitting halves are disconnected.
Dairy Beverage Tubing Kent Elastomer Products serves the food and beverage industry with dairy and beverage tubing products in a variety of FDA NSF and USP Class VI compounds including thermoplastic elastomer TPE thermoplastic vulcanizate. Standards are published in the US Pharmocopeia and the National Formulary USP NF. USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials.
Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal. Our ISO 90012015 compliant and FDA registered facilities produce NSF and USP Class VI food and beverage tubing solutions.

Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Usp Class Vi Foster Corporation

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe

Usp Class Vi O Rings And Seals Eastern Seals Uk Ltd

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Cpc Usp Class Vi Compliant Polysulfone High Flow Quick Disconnect Couplings Cole Parmer

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

0 75 3 4 Id Fda Usp Class Vi Platinum Silicone W Polyester Braid Food And Pharma Grade Flex Technologies Incorporated
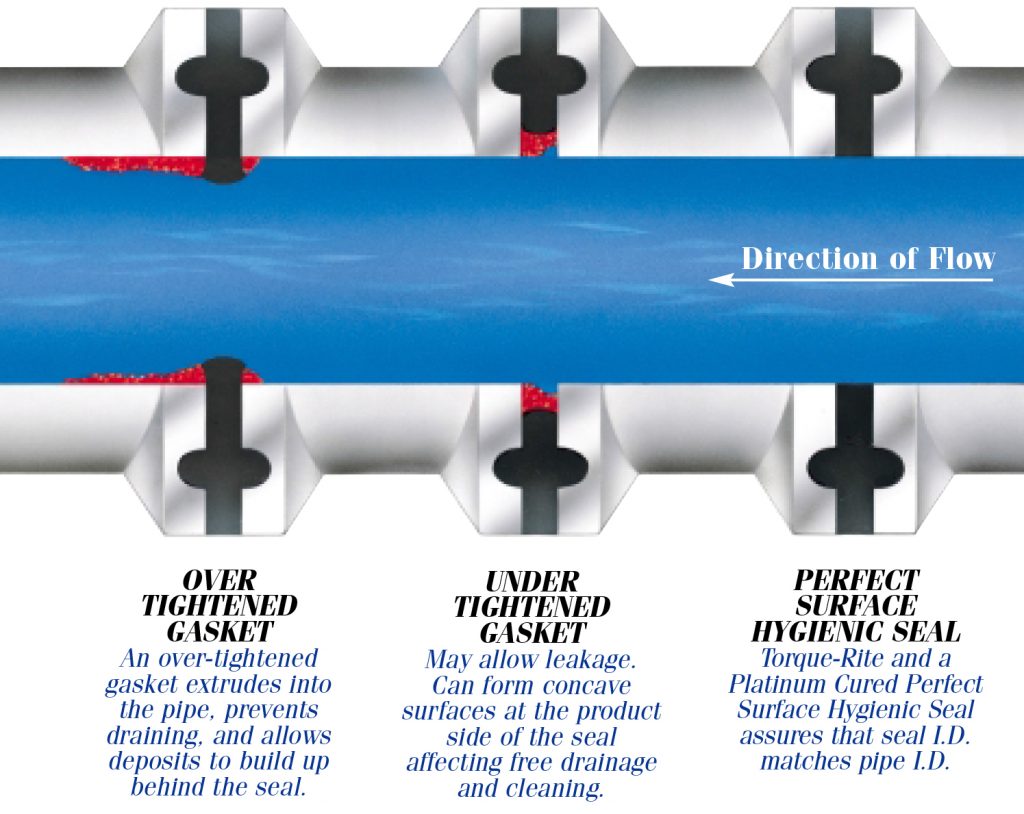
Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

What Is Usp Class Vi Testing Tbl Plastics